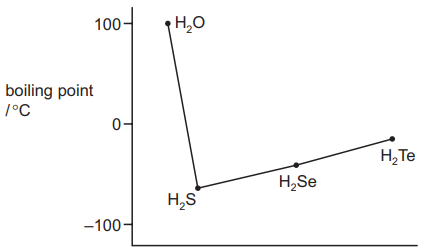
The graph shows the boiling points of the hydrogen compounds of Group 16 elements.
Which statement correctly explains why water does not fit the trend of the other compounds?
1 )
There are fewer electrons in the oxygen atoms so there is less shielding of the nuclear charge.
There are strong hydrogen bonds in water but not in the other compounds.
3 )
The covalent bonds in water are much stronger than in the other compounds.
4 )
The water molecules are smaller and so have stronger van der Waals’ forces.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!











