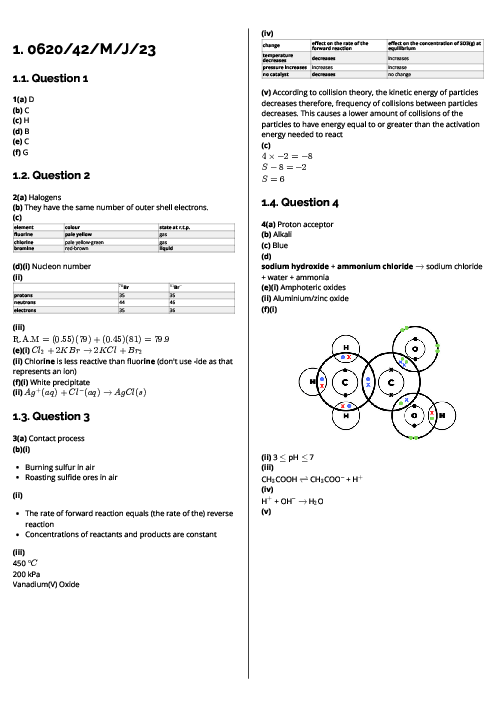
Lumps of calcium carbonate react with dilute hydrochloric acid as shown.
CaCO$_3$ + 2HCl → CaCl$_2$ + H$_2$O + CO$_2$
Which change in conditions decreases the rate of the reaction?
1 )
increasing the concentration of the acid
2 )
increasing the volume of the acid
increasing the size of the lumps of calcium carbonate
4 )
increasing the temperature
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!