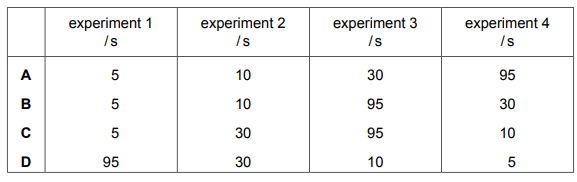
A student carries out four experiments to investigate the rate of reaction between 3.0 g of
calcium carbonate and hydrochloric acid.
$CaC{O_3}\left( s \right) + 2HCl\left( {aq} \right) \to CaC{l_2}\left( {aq} \right) + C{O_2}\left( g \right) + {H_2}O\left( l \right)$
experiment 1 $CaC{O_3}powder + 2.0mol\,d{m^{ - 3}}HCL\,at\,{35^ \circ }C$
experiment 2 $CaC{O_3}powder + 2.0mol\,d{m^{ - 3}}HCL\,at\,{35^ \circ }C$
experiment 3 $l\arg e\,chips\,of\,CaC{O_3} + 1.0mol\,d{m^{ - 3}}HCL\,at\,room\,temperature$
experiment 4 $l\arg e\,chips\,of\,CaC{O_3} + 1.0mol\,d{m^{ - 3}}HCL\,at\,{35^ \circ }C$
The student collects the $C{O_2}\left( g \right)$ and times how long it takes to produce the same volume of gas for each experiment.
What could be the correct times for the four experiments?
1 )
A
B
3 )
C
4 )
D
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!











