Using the particle model, how does increasing temperature affect the rate at which a solute dissolves in a solvent?
1 )
Higher temperature causes solute particles to become smaller, allowing them to fit between solvent particles more easily
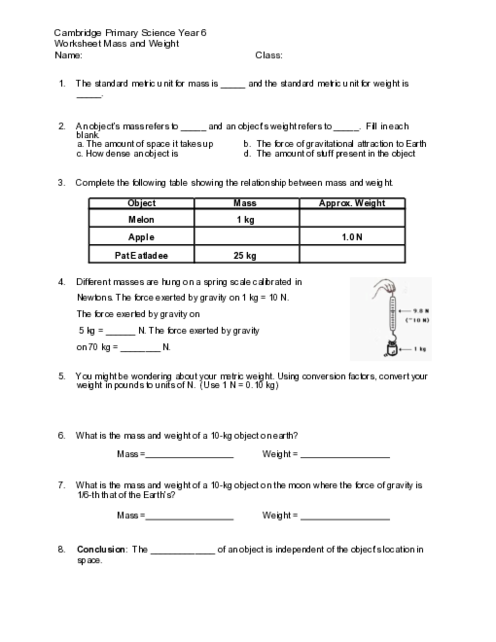
Higher temperature adds energy to particles, causing them to move faster and spread more quickly through the solvent
3 )
Higher temperature increases the mass of the solvent, allowing it to hold more solute particles
4 )
Higher temperature changes the chemical properties of the solute, making it more attractive to solvent particles
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!