The diagram shows a gas syringe with a free-moving piston. The syringe contains gaseous hydrogen, gaseous iodine and gaseous hydrogen iodide at equilibrium.
Three changes are listed.
1- increasing the total pressure by adding an inert gas and keeping the volume constant
2- increasing the pressure by adding more gaseous hydrogen iodide and keeping the volume constant
3- decreasing the volume by pushing the piston to the left
Which changes will result in an equilibrium position at which the rate of the forward reaction has increased?
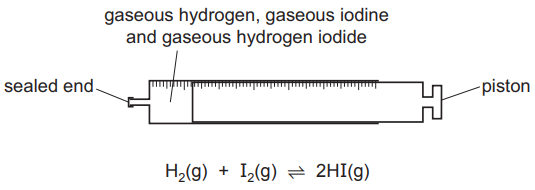
1 )
2 only
2 )
1 and 2
3 )
1 and 3
2 and 3
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!