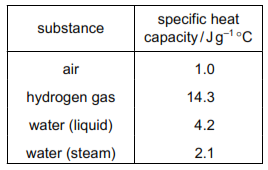
The specific heat capacity of different substances is shown.
Which statement is correct?
1 )
Air is a more stable environment than water because it is more resistant to changes in temperature.
It takes more energy to raise the temperature of hydrogen gas than it does to raise the temperature of water.
3 )
A specific heat capacity of 4.2 J g–1 °C means that it takes 4.2 J of energy to vaporise 1.0 g of liquid water.
4 )
There are more hydrogen bonds between water molecules in a gas than between water molecules in a liquid.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!