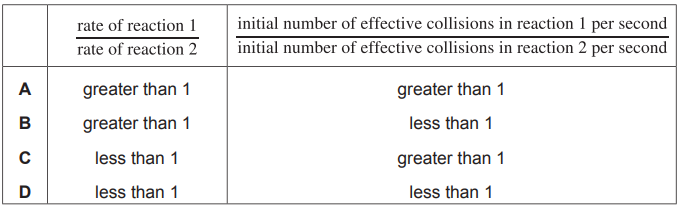
In reaction 1, a student measures the initial rate of production of CO$_2$(g) when CuCo$_3$(s) is added to 50cm$^3$ of 0.1 mol dm$^{- 3}$ HNO$_3$(aq).
In reaction 2, the student repeats the experiment using 50cm$^3$ of 0.5mol dm$^{ - 3}$ HNO$_3$(aq) and the same mass of CuCo$_3$(s).
In reaction 1 and reaction 2, the acid is in excess and samples of the same CuCo$_3$ powder are used.
Which row is correct?
1 )
A
2 )
B
3 )
C
D
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!











