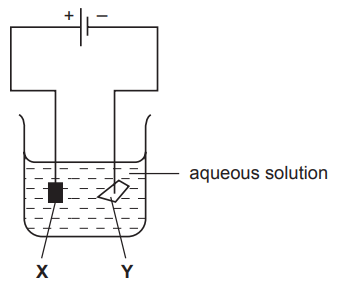
The diagram shows an electrolysis experiment using metals X and Y as electrodes.
One of the metals becomes coated with copper.
Which metal becomes coated and which aqueous solution is used?
1 )
metal: X / aqueous solution: $CrC{l_3}$
2 )
metal: X / aqueous solution: $CuC{l_2}$
3 )
metal: Y / aqueous solution: $CrC{l_3}$
metal: Y / aqueous solution: $CuC{l_2}$
تحلیل ویدئویی تست
تحلیل ویدئویی برای این تست ثبت نشده است!