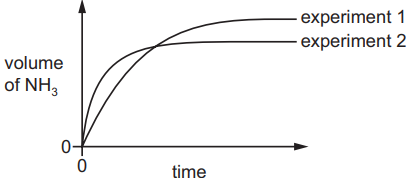
The volume of ammonia produced against time is measured in two experiments.
N$_2$(g) + 3H$_2$(g) $\rightleftharpoons$ 2NH$_3$(g) ΔH = -92 kJ mol$^{-1}$
In experiment 1, 3 mol of H$_2$(g) and 1 mol of N$_2$(g) react together at 45$^\circ$ C and a pressure of 200 atm.
A graph showing the volume of ammonia produced against time is plotted.
Experiment 2 is then performed. Experiment 2 differs from experiment 1 in one condition only.
How does experiment 2 differ from experiment 1?
1 )
An iron catalyst is present in experiment 2.
2 )
2 mol of helium gas is present in the reaction mixture in experiment 2.
3 )
A pressure of 250 atm is used in experiment 2.
A temperature of 600$^\circ$ C is used in experiment 2.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!