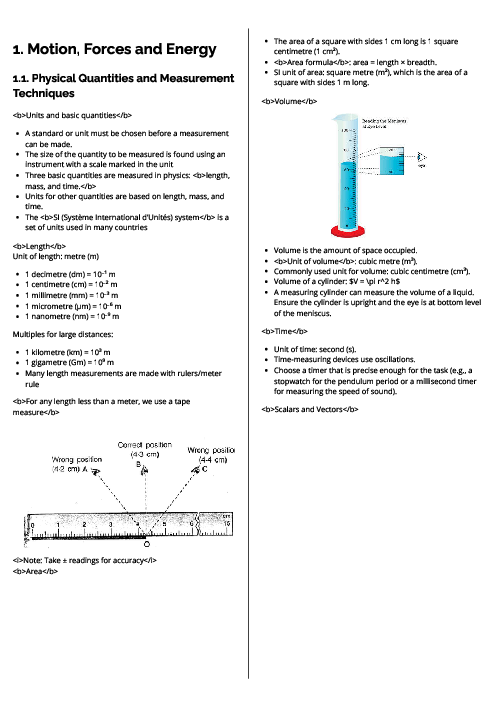
The nuclide notation of the isotope strontium-90 is $^{90}_{38}Sr$.
Which statement is correct?
1 )
A nucleus of strontium-90 has 38 neutrons.
A nucleus of strontium-90 has 52 neutrons.
3 )
A nucleus of strontium-90 has 90 electrons.
4 )
A nucleus of strontium-90 has 90 neutrons.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!