Arsenic forms a compound with fluorine. In this compound, the arsenic atom has no lone pair of electrons and there are no dative bonds.
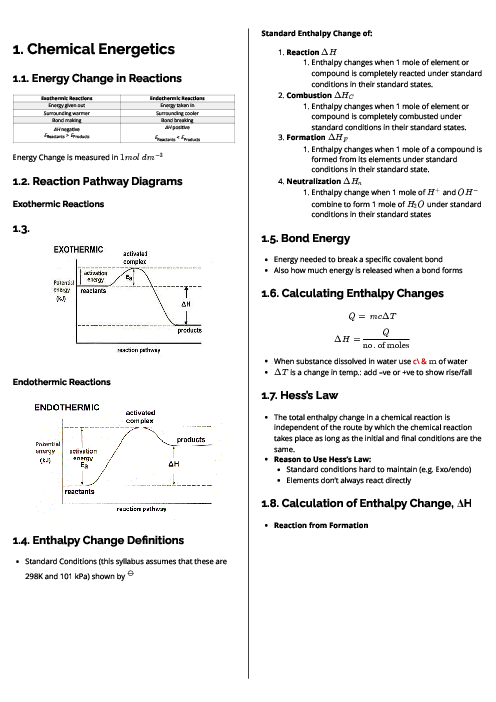
Selenium also forms a compound with fluorine. In this compound, the selenium atom has no lone pair of electrons and there are no dative bonds.
In which compounds are there two different bond angles?
(In this question, 180$^\circ$ bond angles should be ignored.)
1 )
both arsenic fluoride and selenium fluoride
arsenic fluoride only
3 )
selenium fluoride only
4 )
neither arsenic fluoride nor selenium fluoride
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!