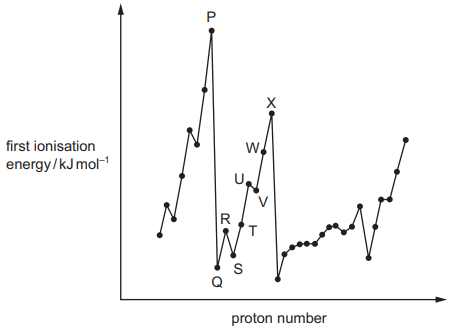
The graph shows the variation of the first ionisation energy with proton number for some elements. The letters used are not the actual symbols for the elements.
Which statement about the elements is correct?
1 )
P and X are in the same period in the Periodic Table.
2 )
The general increase from Q to X is due to increasing atomic radius.
3 )
The small decrease from R to S is due to decreased shielding.
The small decrease from U to V is due to repulsion between paired electrons.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!