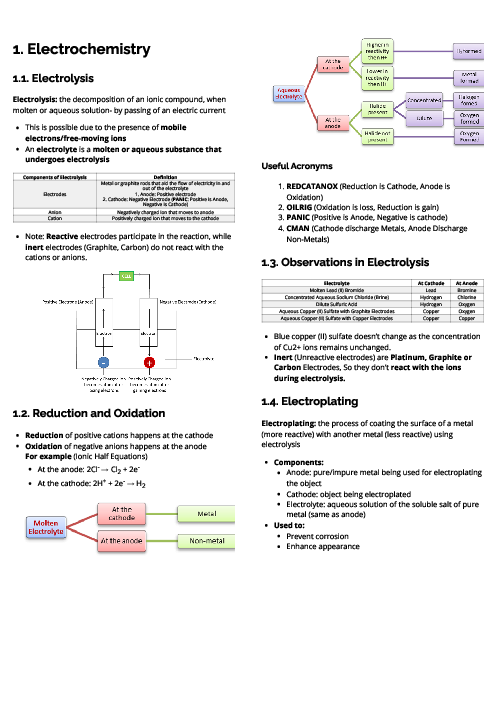
Zinc reacts slowly with dilute sulfuric acid at room temperature.
Bubbles of a gas, L, form on the surface of the zinc.
When a small amount of copper is added, the reaction is faster.
Which row identifies L and explains why the reaction is faster?
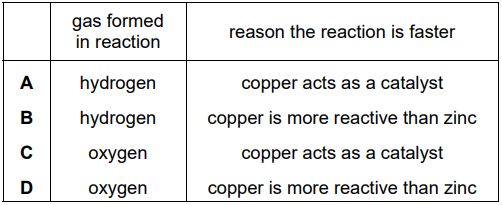
A
2 )
B
3 )
C
4 )
D
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!