The table shows the nucleon number and the number of neutrons in one atom of isotopes W, X, Y and Z.
Which statement about W, X, Y and Z is correct?
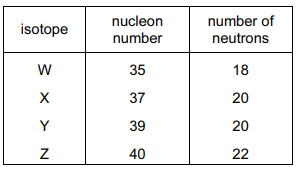
W and X are isotopes of the same element.
2 )
X and Y are isotopes of elements in the same group of the Periodic Table.
3 )
Y and Z are isotopes of elements in the same period of the Periodic Table.
4 )
Z has a higher proton number than Y.
تحلیل ویدئویی تست
تحلیل ویدئویی برای این تست ثبت نشده است!











