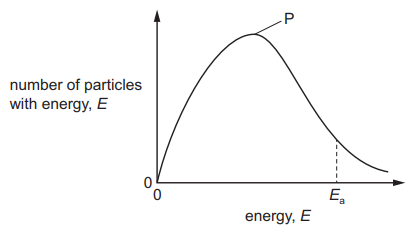
The diagram shows the Boltzmann distribution for one mole of a gas. The gas takes part in a reaction with an activation energy, E$_a$.
Which statement correctly describes the effect of an increase in temperature?
1 )
Peak P will be higher and fewer molecules will have energy > E$_a$.
2 )
Peak P will be higher and more molecules will have energy > E$_a$.
3 )
Peak P will be lower and fewer molecules will have energy > E$_a$.
Peak P will be lower and more molecules will have energy > E$_a$.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!