Molten aluminium chloride has a simple molecular structure. Each molecule consists of two aluminium atoms and six chlorine atoms.
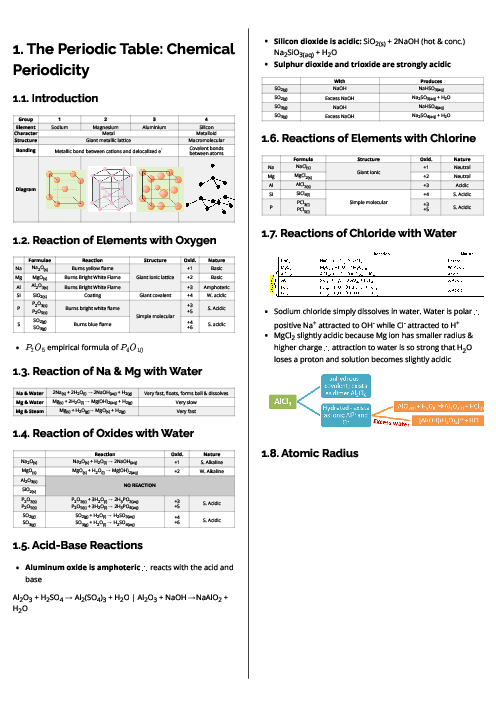
Which statement is correct?
1 )
All the chlorine atoms in 1 g of molten aluminium chloride have the same mass.
2 )
$One{\text{ }}mole{\text{ }}of{\text{ }}molten{\text{ }}aluminium{\text{ }}chloride{\text{ }}contains{\text{ }}6.02{\text{ }} \times {\text{ }}{10^{23}}aluminium{\text{ }}atoms.{\text{ }}$
$One{\text{ }}mole{\text{ }}of{\text{ }}molten{\text{ }}aluminium{\text{ }}chloride{\text{ }}contains{\text{ }}3.61{\text{ }} \times {\text{ }}{10^{24}}chlorine{\text{ }}atoms.$
4 )
$The{\text{ }}empirical{\text{ }}formula{\text{ }}of{\text{ }}molten{\text{ }}aluminium{\text{ }}chloride{\text{ }}is{\text{ }}A{l_2}C{l_6}{\text{ }}.$
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!