A method used to investigate the rate of reaction of calcium carbonate with dilute hydrochloric acid under different conditions is shown.
- place 50 cm$_3$ of dilute hydrochloric acid in a conical flask.
- Add a known volume of water to the conical flask.
- Heat the conical flask to the required temperature.
- Add 1.0 g of calcium carbonate to the conical flask.
- Measure the time taken for the reaction to finish.
Which volume of water and which temperature give the shortest time taken for the reaction to finish?
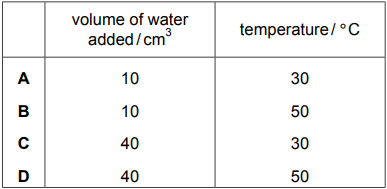
1 )
A
B
3 )
C
4 )
D
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!











