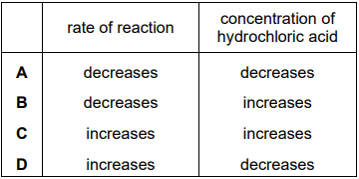
Calcium carbonate reacts with hydrochloric acid.
$CaC{O_3} + 2HCl \to CaC{l_2} + C{O_2} + {H_2}O$
Which row describes how the rate of reaction and the concentration of hydrochloric acid change as the reaction occurs?
1 )
A
2 )
B
C
4 )
D
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!











