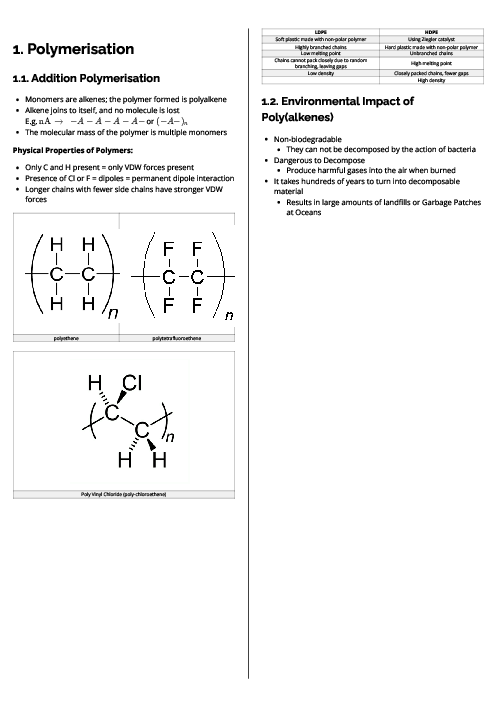
X, and Z are elements all found within Groups 13, 14 and 15 of the Periodic Table.
X is in the same group in the Periodic Table as Y.
Y and Z are in Period 3.
The first ionisation energy of X is greater than the first ionisation energy of Y.
The melting point of Z is less than the melting point of Y.
Y and Z both form chlorides which are white solids. These white solids react with water to produce solutions with a pH of less than 4.
Which row of the table shows the possible identities of X and Y?
A
2 )
B
3 )
C
4 )
D
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!