In the gold foil experiment, most particles passed straight through the foil while a few were deflected. What did this experiment demonstrate about atomic structure?
1 )
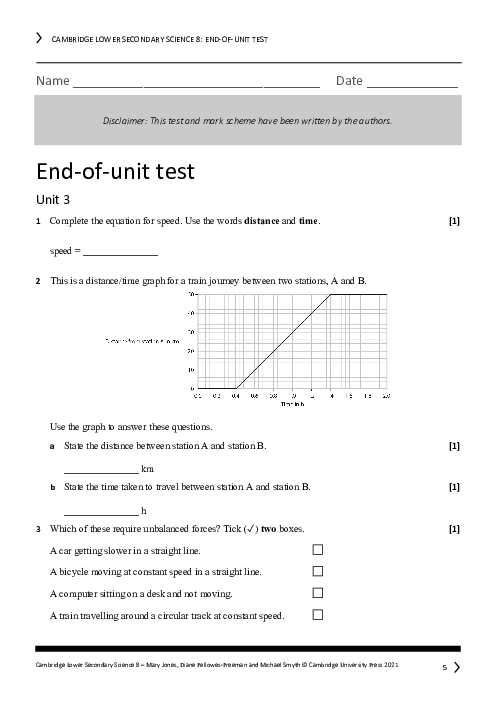
Atoms are solid spheres with electrons scattered throughout
Atoms are mostly empty space with a dense nucleus at the center
3 )
Atoms contain equal numbers of protons and electrons
4 )
Atoms cannot be split into smaller particles
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!