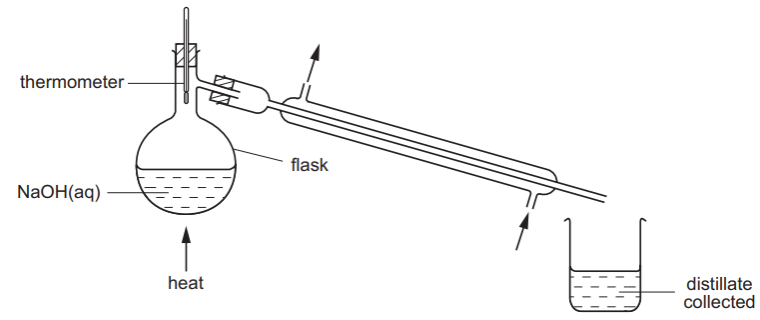
The pH of some aqueous sodium hydroxide is measured. The solution is then distilled as shown.
How do the pH values of the distillate and of the solution left in the flask compare with the original?
1 )
pH of the distillate: higher / pH of the solution left in the flask: higher
2 )
pH of the distillate: higher / pH of the solution left in the flask: lower
pH of the distillate: lower / pH of the solution left in the flask: higher
4 )
pH of the distillate: lower / pH of the solution left in the flask: lower
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!