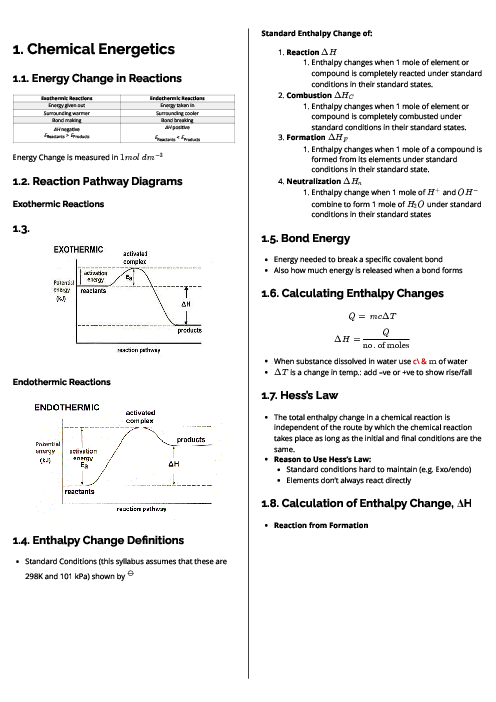
Sodium chromate(VI), Na$_2$CrO$_4$, is manufactured by heating chromite, FeCr$_2$O$_4$, with sodium carbonate in an oxidising atmosphere. Chromite contains Cr$_2$O$_4^{2–}$ ions.
2FeCr$_2$O$_4$ + 4Na$_2$CO$_3$ + 3$\frac{1}{2}$ O$_2$ $\to$ 4Na$_2$CrO$_4$ + Fe$_2$O$_3$ + 4CO$_2$
What happens in this reaction?
Chromium and iron are the only elements oxidised.
2 )
Chromium, iron and carbon are oxidised.
3 )
Only chromium is oxidised.
4 )
Only iron is oxidised.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!