Bromocresol green is an acid-base indicator. Below a pH of 3.8 it is yellow. Above a pH of 5.4 it is blue. Between these values it is green.
Bromocresol green is added to the aqueous solution formed when the chloride of element T is added to water. The colour becomes yellow.
When an excess of the solid oxide of element U is slowly added to this yellow solution, the indicator turns green then blue.
Which row could identify element T and element U?
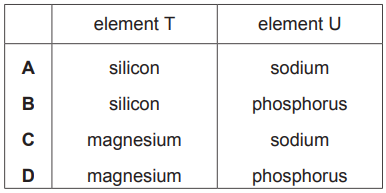
A
2 )
B
3 )
C
4 )
D
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!











