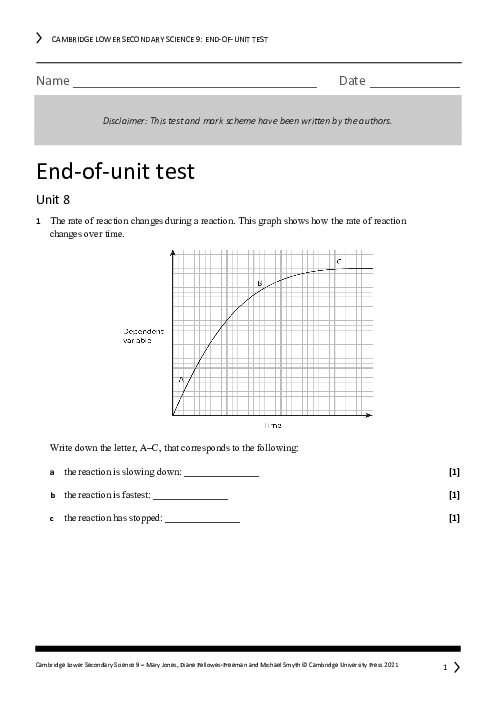
Which of the following correctly describes the trend in reactivity as you move down Group 1 (alkali metals)?
1 )
Reactivity decreases because atoms become smaller
Reactivity increases because the outer electron is more easily lost
3 )
Reactivity remains constant across the group
4 )
Reactivity decreases because the outer electron is held more tightly
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!