In the high temperatures of car engines, nitrogen reacts with oxygen to produce nitrogen monoxide.
$\frac{1}{2}$N$_2$(g) + $\frac{1}{2}$O$_2$(g) → NO(g) ΔH$^\ominus$ = +90kJ mol$^{-1}$
This reaction has activation energy E$_a$.
Which reaction pathway diagram correctly represents this reaction?
1 )
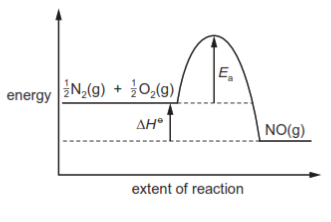
2 )
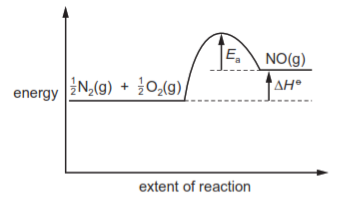
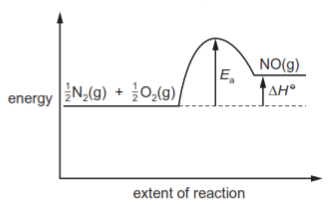
4 )
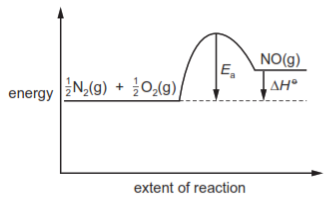
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!