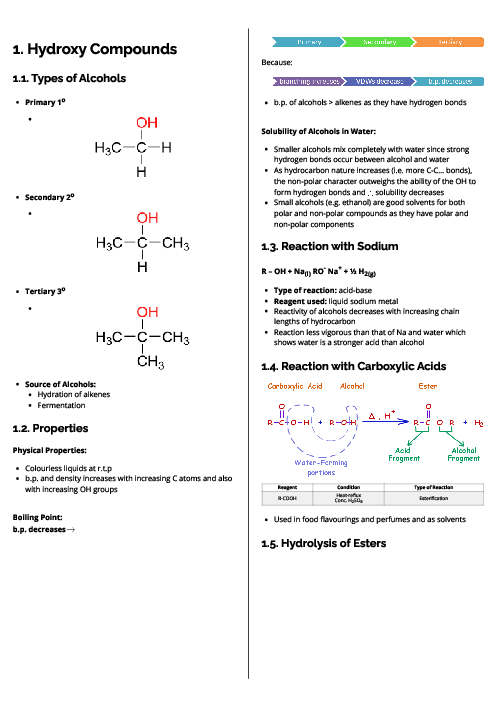
0.200 mol of sulfur dioxide and 0.200 mol of oxygen are placed in a 1.00 dm$^3$ sealed container. The gases are allowed to react until equilibrium is reached.
2SO$_2$ + O$_2$ $\rightleftharpoons$ 2SO$_3$
At equilibrium there is 0.100 mol of SO$_3$ in the container.
What is the value of K$_c$?
1 )
0.150 mol dm$^{–3}$
2 )
0.800 mol dm$^{–3}$
3 )
1.25 mol$^{–1}$ dm$^3$
6.67 mol$^{–1}$ dm$^3$
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!