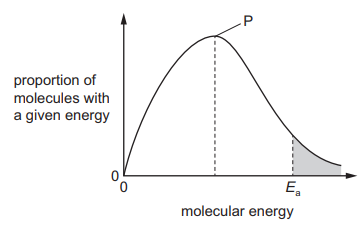
The diagram shows the Boltzmann distribution of energies in a gas. The gas can take part in a reaction with an activation energy, ${E_a}$. The gas is maintained at a constant temperature.
Which statement is correct?
1 )
If a catalyst is added, peak P will be lower and ${E_a}$ will move to the left.
2 )
If a catalyst is added, peak P will be lower and ${E_a}$ will move to the right.
If a catalyst is added, peak P will be the same and ${E_a}$ will move to the left.
4 )
If a catalyst is added, peak P will be the same and ${E_a}$ will move to the right.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!