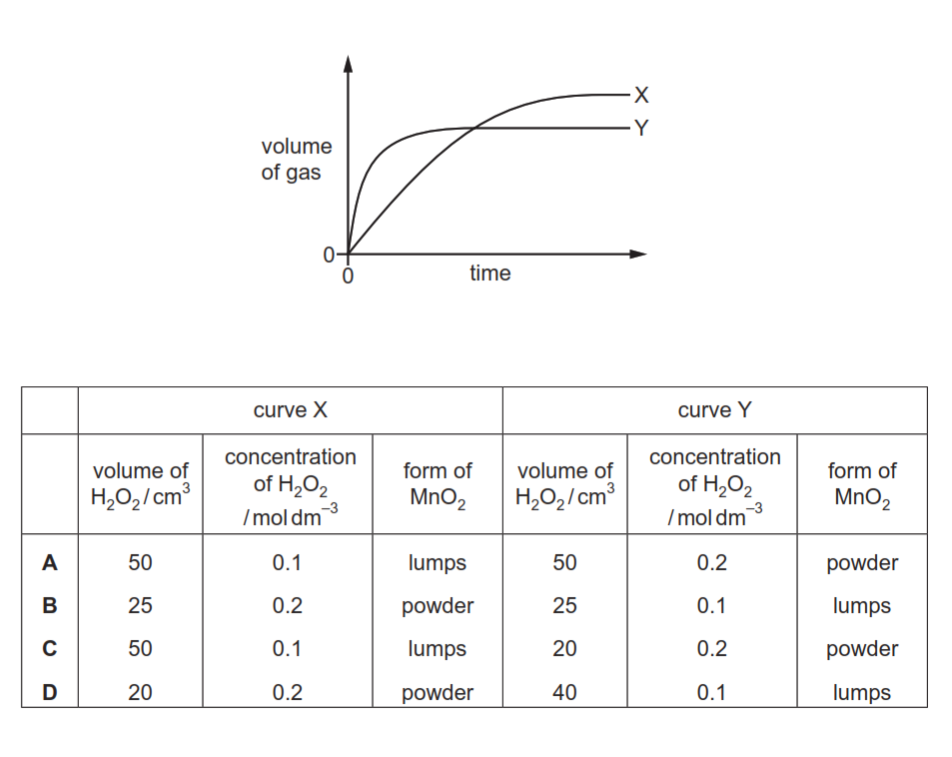
The decomposition of hydrogen peroxide in the presence of MnO$_2$ produces water and oxygen gas.
2H$_2$O$_2$(aq) $→$ 2H$_2$O(l) + O$_2$(g)
The volume of gas collected when 0.2 g of MnO$_2$ is added to two different hydrogen peroxide solutions at 20°C is shown on the graph as curves X and Y.
Which row shows the conditions that will result in curves X and Y?
1 )
A
2 )
B
C
4 )
D
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!