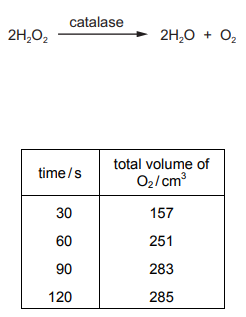
Yeast contains the enzyme catalase which catalyses the breakdown of hydrogen peroxide $\left( {{H_2}{O_2}} \right)$ as shown.
Yeast was added to a solution of hydrogen peroxide and the total volume of oxygen released was
recorded every 30 seconds for 2 minutes. All other variables were standardised.
The data is shown in the table.
What explains the pattern of the data?
1 )
The rate of reaction increases as more enzyme–substrate complexes are formed.
2 )
The rate of reaction increases as the enzyme reaches its maximum velocity $\left( {{V_{max}}} \right)$.
3 )
The volume of oxygen released decreases as the enzymes begin to denature.
The volume of oxygen released decreases as more substrate is converted into product.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!