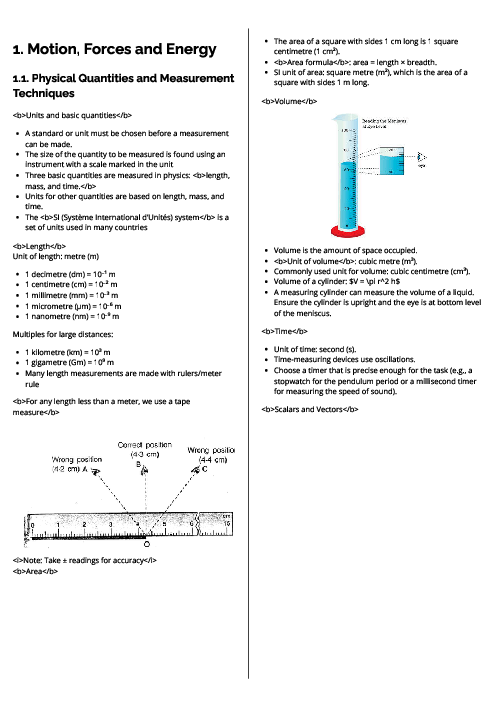
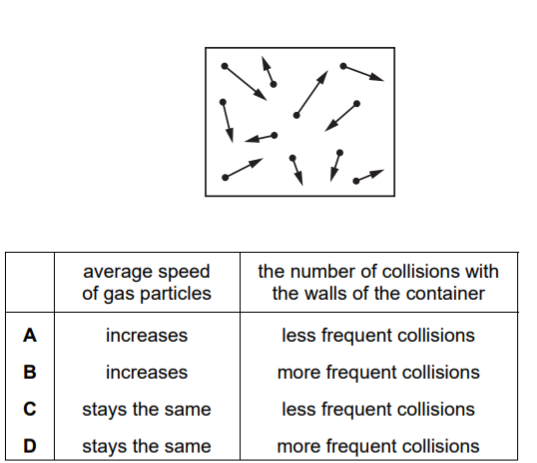
The diagram represents gas particles moving around in a sealed container.
The gas particles collide with the walls of the container.
The temperature of the gas is increased.
What happens to the average speed of the gas particles and what happens to the number of collisions by the gas particles with the walls of the container?
1 )
A
B
3 )
C
4 )
D
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!