Gas Q decomposes slowly at room temperature.
Q(g) $\to$ X(g) + Z(g)
The Boltzmann distribution curve for gas Q at room temperature is shown.
Which change occurs when a catalyst is added to gas Q?
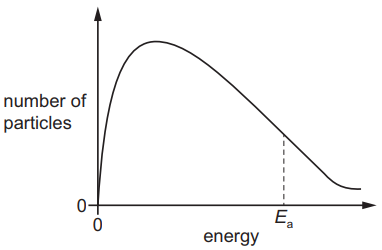
1 )
The peak of the curve moves to the right on the diagram.
The number of particles with enough energy to decompose increases.
3 )
The kinetic energy of the unreacted particles increases.
4 )
The value of E$_a$ decreases, moving the vertical dotted line to the right on the diagram.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!










