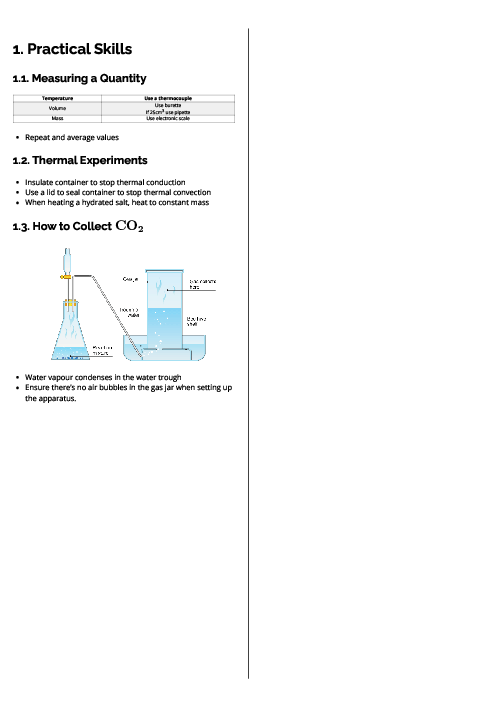
A mixture of the three gases, oxygen, nitrogen and argon, is at a total pressure of 500 kPa. There is a total of 1.2 moles of gas in the mixture.
If the oxygen gas alone occupied the entire volume of the mixture, it would exert a pressure of 150 kPa.
At room conditions the amount of nitrogen gas in the mixture would occupy a volume of 5.76 dm$^3$.
What is the partial pressure of the argon gas in the mixture?
1 )
150 kPa
2 )
200 kPa
250 kPa
4 )
300 kPa
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!