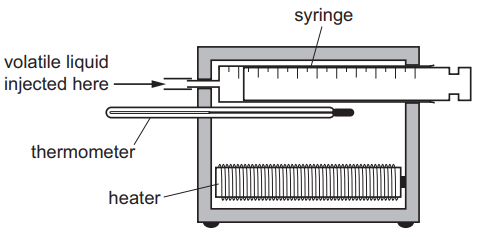
The diagram shows the apparatus used to find the relative molecular mass of a volatile liquid.
When 0.10 g of a volatile liquid is injected into the syringe, all of the volatile liquid evaporates and the volume increases by 85 cm$^3$.
The heater maintains a temperature of 400K and the experiment is carried out at a pressure of 101 300Pa.
If the vapour of the volatile liquid behaves as an ideal gas, which expression can be used to calculate the relative molecular mass of the liquid?
1 )
M$_r$ = (85 × 101 300) $\div$ (0.10 × 8.31 × 400)
2 )
M$_r$ = (85 × 101.3) $\div$ (0.10 × 8.31 × 400)
M$_r$ = (0.10 × 8.31 × 400) $\div$ (85 × 10$^{–6}$ × 101 300)
4 )
M$_r$ = (0.10 × 8.31 × 400) $\div$ (85 × 10$^{–6}$ × 101.3)
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!











