Which statement about elements in the Periodic Table is correct?
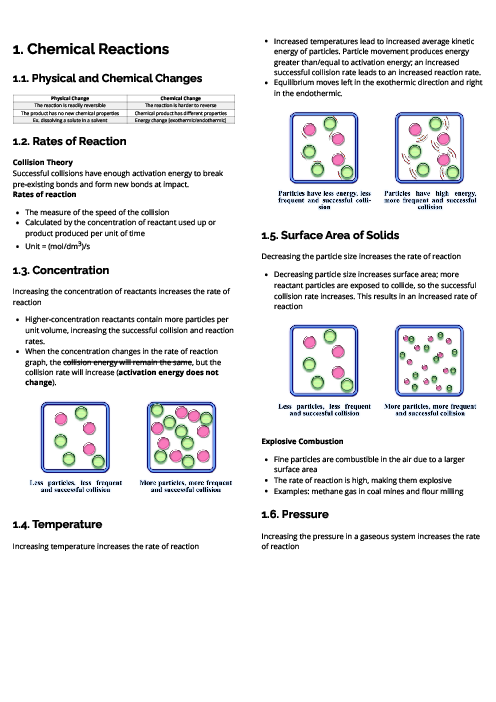
A potassium ion, K$^+$, has the same electronic configuration as a chloride ion, Cr$^-$.
2 )
The electronic configuration of a Ca$^{2+}$ ion is 2,8,8,2.
3 )
The halogens are in Group VI and so their atoms have six electrons in their outer shell.
4 )
Magnesium is in Period 3 and so a magnesium ion, Mg$^{2+}$, has three occupied electron shells.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!