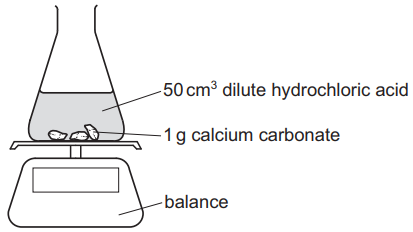
An experiment is set up as shown.
The mass of the conical flask and its contents is measured at 30-second intervals.
Which statement about the reaction and changes to the reaction conditions is correct?
1 )
Adding 10cm$^3$ of water to the 50cm$^3$ of acid increases the rate of the reaction.
2 )
Increasing the size of the pieces of calcium carbonate increases the rate of the reaction.
Increasing the temperature increases the rate of the reaction.
4 )
The mass of the conical flask and its contents increases as carbon dioxide is formed.
تحلیل ویدئویی تست
منتظریم اولین نفر تحلیلش کنه!











